TORONTO, ON / ACCESSWIRE / April 27, 2021 / Theralase® Technologies Inc. ("Theralase" or the "Company") (TSXV:TLT)(OTCQB:TLTFF), a clinical stage pharmaceutical company dedicated to the research and development of light activated Photo Dynamic Compounds ("PDC") and associated drug formulations to safely and effectively destroy various cancers, bacteria and viruses has released the Company's Quarterly Newsletter ("Newsletter") which provides updates on the previous quarter and progress on the Phase II Non-Muscle Invasive Bladder Cancer ("NMIBC") Clinical Study ("Study II").
The Newsletter can be found on the Company's website at www.theralase.com/quarterly-newsletters/
Highlights from the Newsletter:
- Clinical Study Sites ("CSS")
11 CSS's have been launched in Canada (5) and the US (6) for patient enrollment and treatment for Study II. - Patients Treated
Study II has enrolled and provided the primary study treatment for 18 patients (including three patients from the Phase Ib study treated at the Therapeutic Dose) for a total of 21 patients. - Study II Preliminary Results
An analysis of the primary (Complete Response ("CR") at any point in time) and secondary (Duration of CR) objectives, at 90 and 180 days, the CR rate (negative urine cytology and negative cystoscopy) is 33.3% and 28.60%, respectively, while the Partial Response ("PR") rate (positive urine cytology and negative cystoscopy) is 9.5% and 9.5%, respectively, leading to a 42.8% and 38.10%, Total Response rate (CR and PR), respectively.
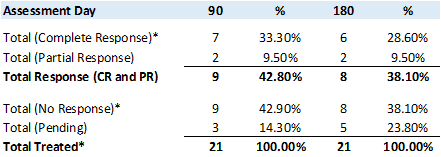
*Includes 3 patients at the Therapeutic Dose from the Phase Ib NMIBC Clinical Study
Comparing the 90 and 180 day assessment data, demonstrates that once a patient has obtained CR or PR, the duration of the response remains fairly durable.
In accordance with FDA guidelines to industry, the patients who have achieved a PR are being further assessed via CT scan and biopsy of the prostatic urethra to determine if upper tract Urothelial Cell Carcinoma ("UCC") or prostatic urethra UCC can be detected to allow these patients to be re-categorized as CR.
An analysis of the tertiary objective (Adverse Events ("AE") > 4, that do not resolve within 450 days) showed that 1 patient, who had a negative cytology (no presence of cancer cells in urine) after a single Study Treatment, experienced a grade 3 (Acute Kidney Injury) that was discovered at the 30 day check-up that was considered unlikely related to the Study Drug, probably related to the Study Procedure and possibly related to the Study Device, by the Principal Investigator ("PI"). This same patient, 5 days later, experienced a grade 5 adverse event (Death due to cardiac arrest) that was considered unlikely related to the Study Drug, unlikely related to the Study Procedure and unlikely related to the Study Device, by the PI. The patient had a complex medical history, including diabetes, cardiovascular disease, as well as suffering from benign prostatic hyperplasia. Based on the patient's history and the timing of the AE, the Company has concluded that both these events were unrelated to the Study Drug and/or Study Device.
- Study II Optimized Treatment Preliminary Results
Commencing August 1, 2020, all new and existing patients enrolled and treated (primary and maintenance Study Treatments) in Study II were treated using the Study II treatment optimizations as communicated via press release on July 30, 2020. In total, 6 patients were treated using the Study II treatment optimizations.
Although very early for a statistical analysis the primary objective at 90 days for patients receiving the optimized primary Study Treatment demonstrates a 33.3% CR rate, with 50.0% data pending and only 16.7% with no response.
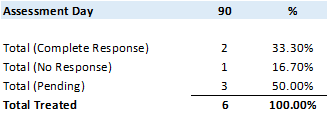
To receive future Newsletters, please contact info@theralase.com
About Theralase® Technologies Inc.
Theralase® is a clinical stage pharmaceutical company dedicated to the research and development of light activated Photo Dynamic Compounds and their associated drug formulations intended to safely and effectively destroy various cancers, bacteria and viruses.
Additional information is available at www.theralase.com and www.sedar.com
Forward Looking Statement:
This news release contains "forward-looking statements" which reflect the current expectations of the Company's management for future growth, results of operations, performance, business prospects and opportunities. Such statements include, but are not limited to, statements regarding the Company's proposed development plans with respect to Photo Dynamic Compounds and their drug formulations. Wherever possible, words such as "may", "would", "could", "should", "will", "anticipate", "believe", "plan", "expect", "intend", "estimate", "potential for" and similar expressions have been used to identify these forward-looking statements. These statements reflect management's beliefs with respect to future events and are based on information currently available to management. Forward-looking statements involve significant risks, uncertainties and assumptions; including, with respect to the ability of the Company to: adequately fund, secure the requisite regulatory approvals to commence and successfully complete a Phase II NMIBC clinical study in a timely fashion and implement its commercialization plans. Many factors could cause the Company's actual results, performance or achievements to be materially different from any future results, performance or achievements that may be expressed or implied by such forward-looking statements; including, without limitation, those listed in the filings made by the Company with the Canadian securities regulatory authorities (which may be viewed at www.sedar.com). Should one or more of these risks or uncertainties materialize or should assumptions underlying the forward-looking statements prove incorrect, actual results, performance or achievements may vary materially from those expressed or implied by the forward-looking statements contained in this news release. These factors should be considered carefully, and prospective investors should not place undue reliance on the forward-looking statements. Although the forward-looking statements contained in the press release are based upon what management currently believes to be reasonable assumptions, the Company cannot assure prospective investors that actual results, performance or achievements will be consistent with these forward-looking statements. The Company disclaims any intention or obligation to revise forward-looking statements whether as a result of new information, future developments or otherwise except as required by law. All forward-looking statements are expressly qualified in their entirety by this cautionary statement.
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
For More Information:
1.866.THE.LASE (843-5273)
416-699-LASE (5273)
www.theralase.com
Kristina Hachey
Chief Financial Officer
khachey@theralase.com
416-699-LASE (5273) x 224
SOURCE: Theralase® Technologies Inc.
View source version on accesswire.com:
https://www.accesswire.com/642333/TheralaseR-Releases-Quarterly-Newsletter






